Core competence ophthalmic surgery
Your expert for mobile treatment chairs
Preventive health care in ophthalmic and microsurgery

Smart technologies
Maximum mobility, ergonomics and
efficiency for your outpatient OR
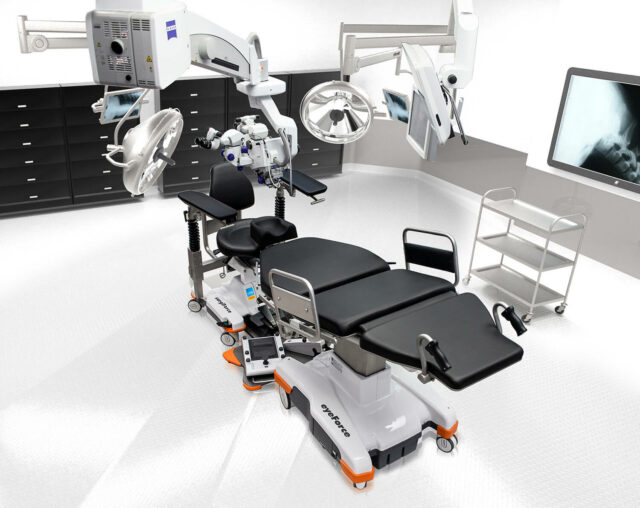
The mobile treatment tables and treatment chairs from UFSK-International OSYS ensure smooth and efficient OR management and create optimal reclining and seating comfort for your staff and patients. Our innovative products have been specially developed for outpatient eye surgery. With their outstanding mobility, the customized work platforms handle fast OR cycles with particularly high patient throughput. This increases the efficiency of your OR and minimizes the daily physical strain on your staff.
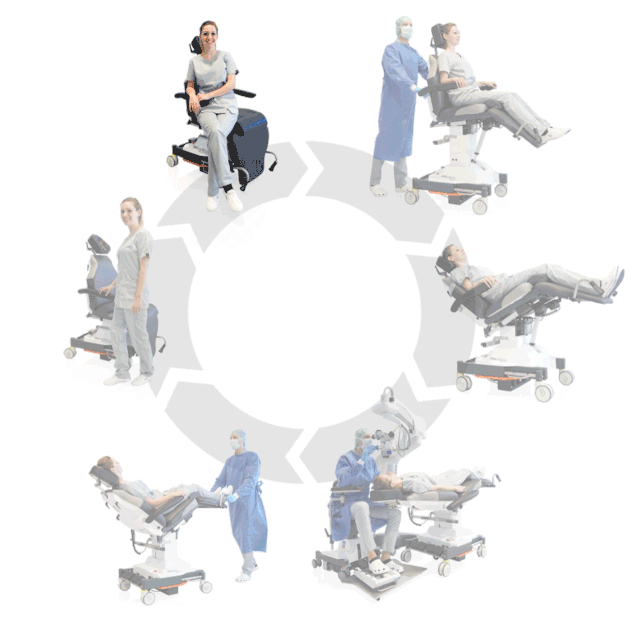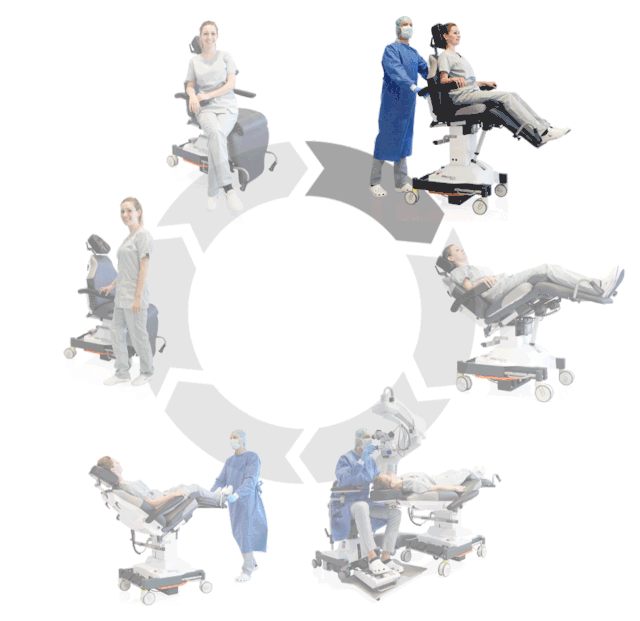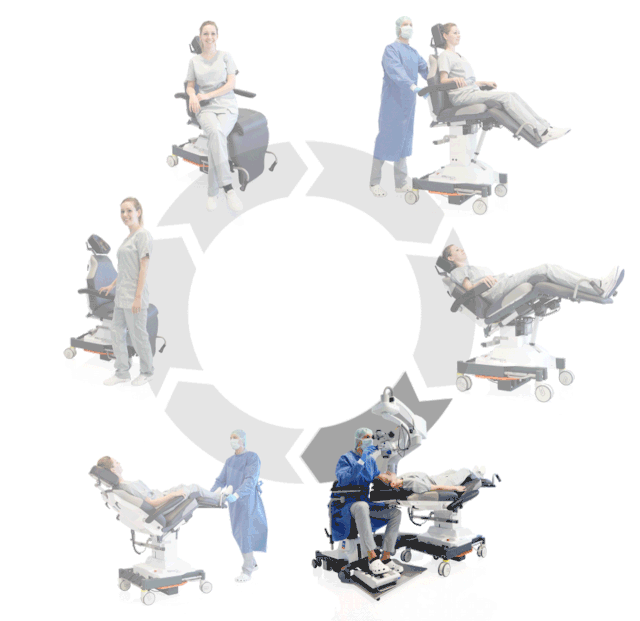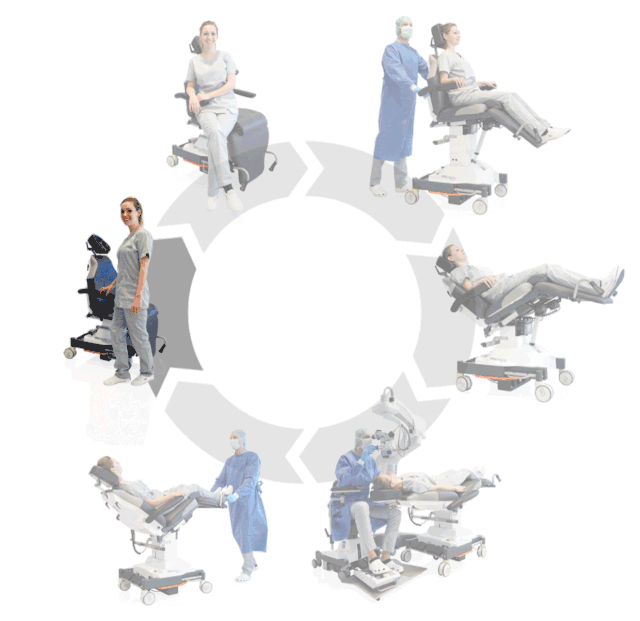
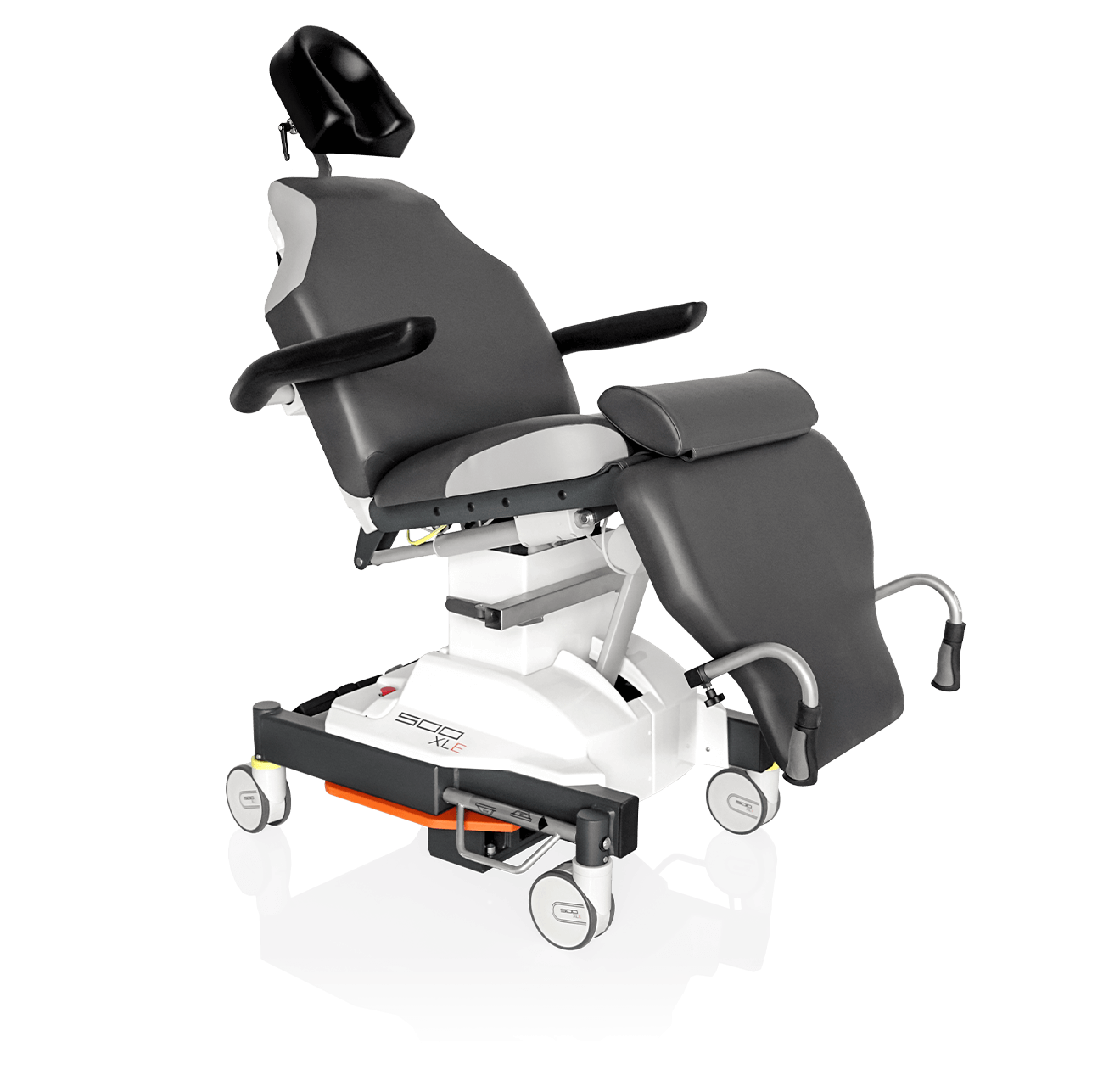
Prepare, operate, wake up – all on one chair
Surgery rotation
Make your OR management even more efficient and economical! The mobile treatment chairs from UFSK-International OSYS were specially designed for time- and cost-saving surgery rotation. The use of our medical treatment chairs enables the simultaneous treatment of several patients without the need for additional time and energy for their repositioning. This means your patients can be prepared and operated on in one chair – and wake up relaxed afterwards.
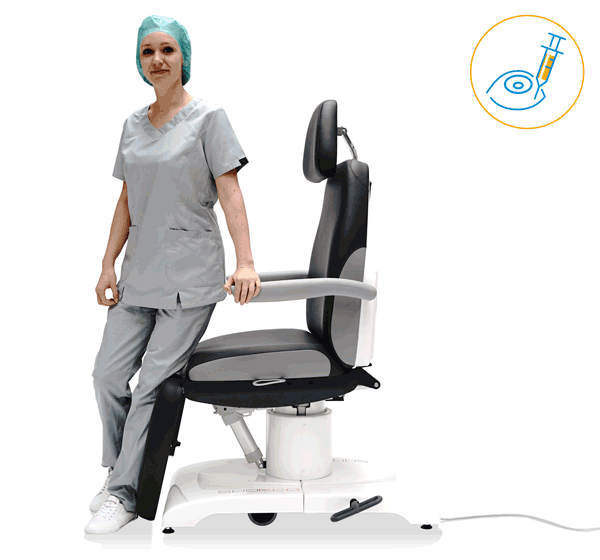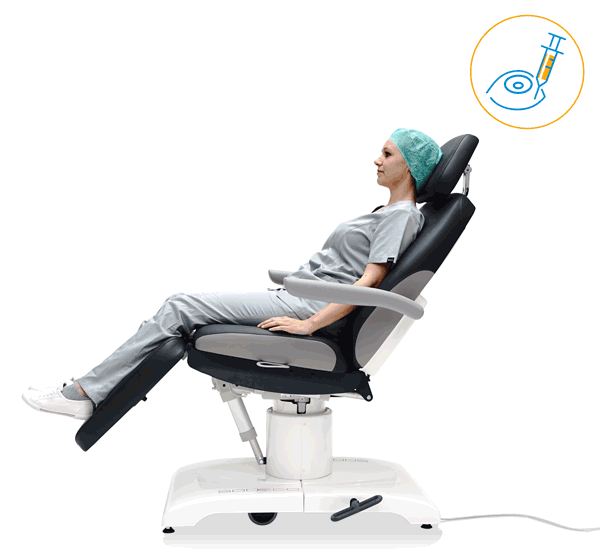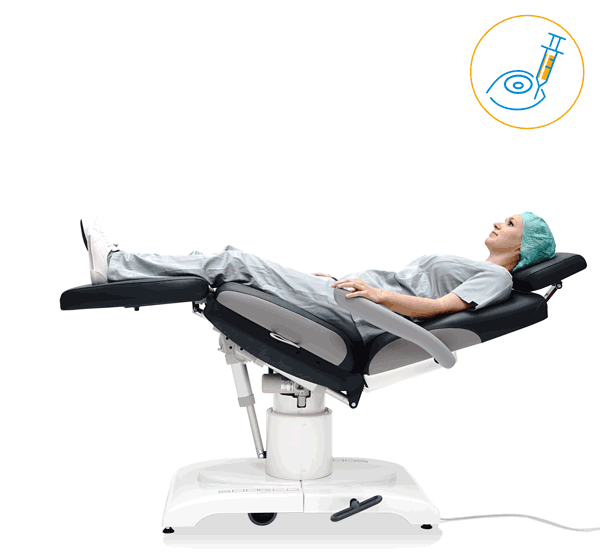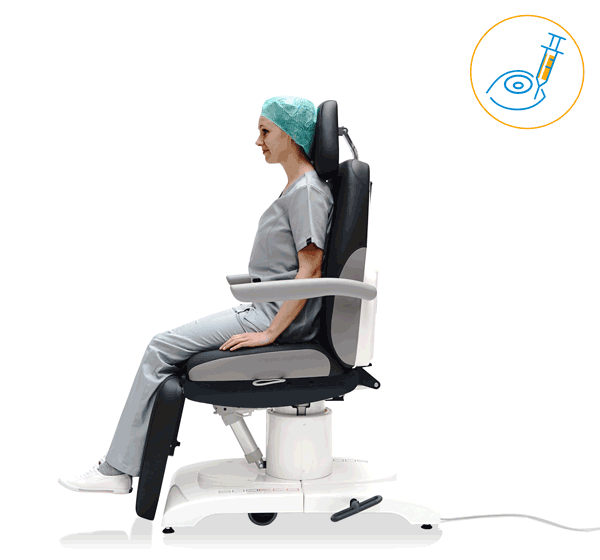
Our treatment chair for walk-in OR concepts
Just Walk-In OR
The rotation principle is already part of daily business in your OR? Then our 500 ECO treatment chair is the perfect addition to your OR system. Thanks to its high adjustment speed, the treatment chair is ready for use in seconds and is thus ideally suited for intravitreal surgical measures. This saves time and increases your average patient throughput for I.V.I. treatments.

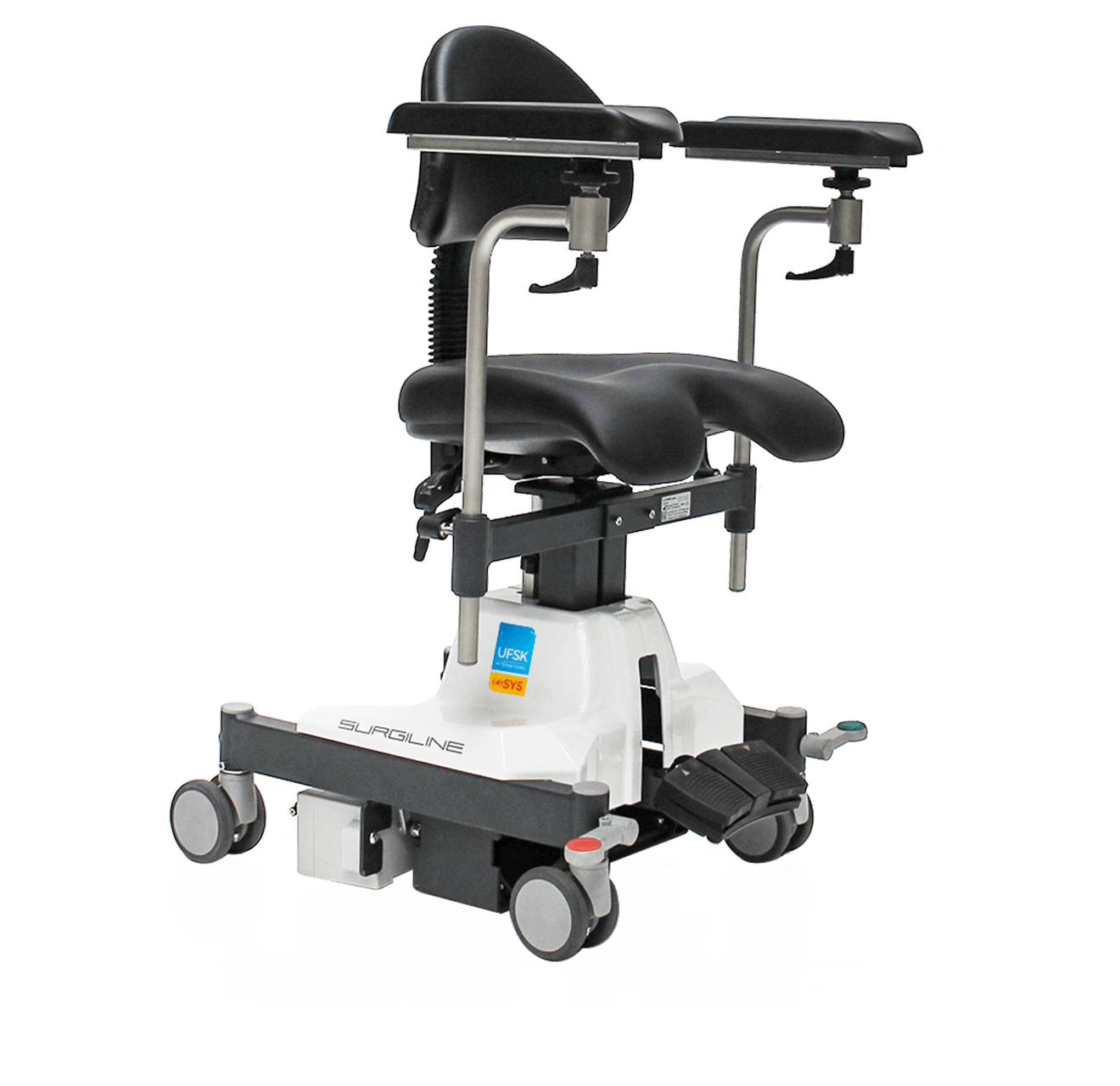
Preventing discomfort
Preventive technologies
The higher the number of OR cycles, the more distance your staff has to cover every day. With the ergonomic products of UFSK-International OSYS you sustainably relieve your staff and thus contribute to improved productivity and a lower level of sickness. The technical refinements of our transport and treatment chairs enable a gentle working method and effectively improve the ability of your OR staff to concentrate.

UFSK-International OSYS
Since 1994 from practice for practice
From our headquarters in Regensburg, Germany, we have been developing mobile operating room equipment for ophthalmic surgery and microsurgery together with internationally renowned ophthalmic surgeons for almost 30 years.
• Customized product development for ophthalmic surgery
• Worldwide service and support
• Over 15,000 UFSK-International OSYS operating systems worldwide
Customer focus and support
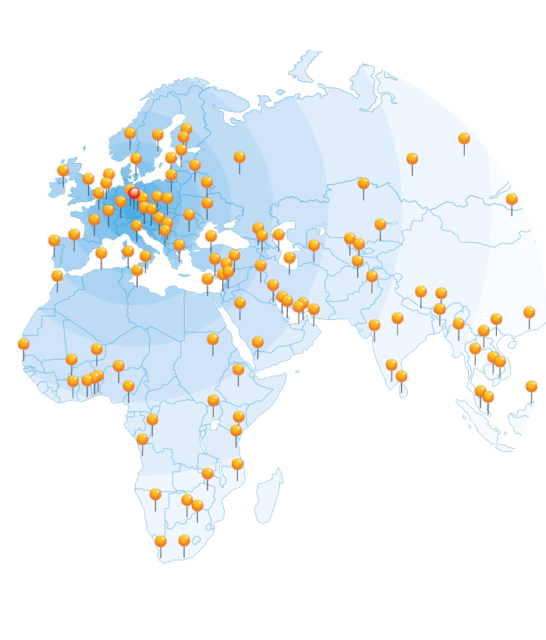
Worldwide sales
and service network
We are there when you need us. With our global sales and service network, we flexibly adapt to your individual requirements. Our personal and reliable support ranges from purchase advice to commissioning and maintenance of UFSK-International OSYS products throughout their entire life cycle.

Quality management
Globally certified
To guarantee the safety of our customers and their patients, we only settle for the highest quality – in all areas of our company. All our products are certified worldwide and are designed, engineered and manufactured in Germany.